Quantum numbers are essential for understanding the intricate details of atomic structure. They serve as a set of numerical values that describe various properties of electrons in atoms. By delving into the world of quantum numbers, we can gain insights into electron configurations, chemical bonding, and the behavior of elements. This article will explore the four types of quantum numbers, their significance, and how they help us understand atomic structure.
What Are Quantum Numbers?
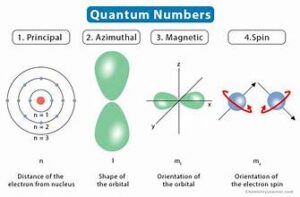
Quantum numbers are a set of four values used to describe the state of an electron in an atom. Each quantum number provides a different piece of information about the electron’s position, energy, and orbital characteristics. The four quantum numbers are:
- Principal Quantum Number (n)
- Angular Momentum Quantum Number (l)
- Magnetic Quantum Number (m_l)
- Spin Quantum Number (m_s)
1. Principal Quantum Number (n)
The principal quantum number, denoted by nnn, indicates the main energy level or shell of an electron in an atom. It essentially determines the size and energy of the orbital where the electron is likely to be found. The principal quantum number can have positive integer values (1, 2, 3, …), with higher values corresponding to higher energy levels and larger orbitals.
- Role in Atomic Structure: The principal quantum number defines the electron’s energy level and its distance from the nucleus. For example, electrons in the first shell (n=1) are closest to the nucleus, while electrons in higher shells are further away.
2. Angular Momentum Quantum Number (l)
The angular momentum quantum number, denoted by lll, describes the shape of the orbital where the electron resides. It can have integer values from 0 to n−1n-1n−1. Each value of lll corresponds to a different type of orbital:
- l=0l = 0l=0: s orbital (spherical shape)
- l=1l = 1l=1: p orbital (dumbbell shape)
- l=2l = 2l=2: d orbital (cloverleaf shape)
- l=3l = 3l=3: f orbital (complex shape)
- Role in Atomic Structure: The angular momentum quantum number determines the orbital’s shape, influencing how electrons are distributed around the nucleus and how they interact with other atoms.
3. Magnetic Quantum Number (m_l)
The magnetic quantum number, denoted by mlm_lml, specifies the orientation of the orbital within a given energy level and shape. It can take on integer values between −l-l−l and +l+l+l, including zero.
- Role in Atomic Structure: The magnetic quantum number affects the orientation of orbitals in space. For example, in the p orbitals (l=1), mlm_lml can be -1, 0, or +1, resulting in three different orientations.
4. Spin Quantum Number (m_s)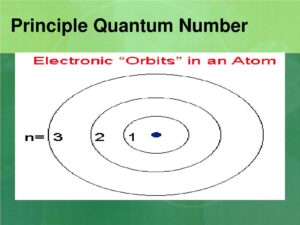
The spin quantum number, denoted by msm_sms, describes the intrinsic angular momentum or spin of the electron. It can have one of two values: +1/2 or -1/2.
- Role in Atomic Structure: The spin quantum number determines the direction of the electron’s spin, which affects its magnetic properties. Electrons with opposite spins can occupy the same orbital, adhering to the Pauli Exclusion Principle.
Significance of Quantum Numbers in Atomic Structure
Quantum numbers provide a comprehensive framework for understanding electron configurations, which are crucial for predicting the chemical properties of elements. Here’s how quantum numbers contribute to our understanding of atomic structure:
- Electron Configuration: Quantum numbers help in determining the arrangement of electrons in an atom. For example, the electron configuration of carbon (1s² 2s² 2p²) is derived from its quantum numbers, indicating that carbon has two electrons in the first shell and four electrons in the second shell.
- Periodic Table Trends: The arrangement of elements in the periodic table reflects their quantum numbers. For example, elements in the same group have similar valence electron configurations, which is explained by their principal and angular momentum quantum numbers.
- Chemical Bonding: Quantum numbers are vital in understanding how atoms bond. The overlap of orbitals and the sharing of electrons between atoms are governed by quantum numbers. This helps in explaining various bonding theories, such as covalent and ionic bonding.
- Spectroscopy and Energy Levels: Quantum numbers are used to describe the energy levels of electrons, which is crucial in spectroscopy. When electrons transition between different energy levels, they absorb or emit light at specific wavelengths, providing information about the atomic structure.
Conclusion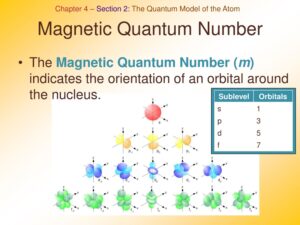
Understanding quantum numbers is fundamental to unlocking the mysteries of atomic structure. These numerical values provide detailed information about the position, energy, and behavior of electrons in an atom. By grasping the significance of the principal, angular momentum, magnetic, and spin quantum numbers, we can better understand electron configurations, chemical bonding, and the underlying principles that govern atomic interactions. This knowledge not only enriches our comprehension of chemistry but also has practical applications in fields ranging from materials science to quantum computing.



