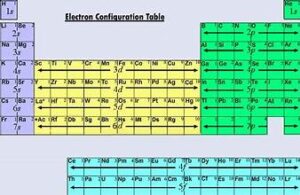
Introduction
Quantum numbers are fundamental to understanding atomic structure and chemistry. They define the properties of electrons in atoms and play a crucial role in determining an element’s electron configuration and, consequently, its chemical behavior. This article will delve into the significance of quantum numbers, their influence on electron configuration, and their impact on chemical properties. By the end, you’ll have a comprehensive understanding of how these abstract concepts shape the nature of elements and their interactions.
What Are Quantum Numbers?
Quantum numbers are a set of values that describe the unique quantum state of an electron in an atom. They are derived from the solutions to the Schrödinger equation, which describes the behavior of electrons in atoms. There are four primary quantum numbers:
- Principal Quantum Number (n)
- Angular Momentum Quantum Number (l)
- Magnetic Quantum Number (m_l)
- Spin Quantum Number (m_s)
Each quantum number provides different information about an electron’s position and energy level within an atom.
1. Principal Quantum Number (n)
The principal quantum number, denoted as nnn, specifies the main energy level or shell of an electron. It is always a positive integer (1, 2, 3, …). The value of nnn determines the overall size and energy of the electron cloud.
- Energy Level: Higher nnn values correspond to higher energy levels and larger electron clouds.
- Distance from Nucleus: Electrons with higher nnn values are located further from the nucleus.
In the context of electron configuration, the principal quantum number dictates the arrangement of electrons into different shells or energy levels.
2. Angular Momentum Quantum Number (l)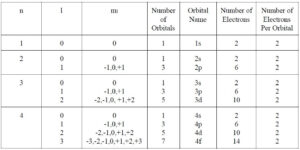
The angular momentum quantum number, denoted as lll, determines the shape of the electron’s orbital. It ranges from 0 to n−1n-1n−1 for each principal quantum number.
- Orbital Shape: Different values of lll correspond to different orbital shapes (s, p, d, f).
- l=0l = 0l=0 corresponds to an s orbital (spherical).
- l=1l = 1l=1 corresponds to a p orbital (dumbbell-shaped).
- l=2l = 2l=2 corresponds to a d orbital (cloverleaf-shaped).
- l=3l = 3l=3 corresponds to an f orbital (complex shapes).
The shape of the orbital affects the spatial distribution of electrons and influences how they interact with other atoms.
3. Magnetic Quantum Number (m_l)
The magnetic quantum number, denoted as mlm_lml, specifies the orientation of the orbital within a given energy level and shape. Its values range from −l-l−l to +l+l+l, including zero.
- Orbital Orientation: For example, in the p orbitals (l=1l = 1l=1), mlm_lml can be -1, 0, or +1, indicating three possible orientations in space.
- Orbital Degeneracy: Different orientations of orbitals have the same energy level in the absence of external fields.
The orientation of orbitals affects how atoms bond and interact with each other.
4. Spin Quantum Number (m_s)
The spin quantum number, denoted as msm_sms, describes the intrinsic angular momentum or spin of an electron. It can have a value of +1/2 or -1/2.
- Electron Spin: This quantum number indicates the direction of the electron’s spin (either clockwise or counterclockwise).
- Pauli Exclusion Principle: No two electrons in an atom can have the same set of four quantum numbers. This principle is essential for determining the electron configuration and stability of atoms.
The spin of an electron contributes to the magnetic properties of an atom and its ability to form bonds.
Influence on Electron Configuration
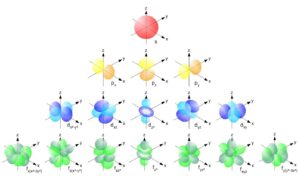
Electron configuration refers to the arrangement of electrons in an atom’s orbitals. Quantum numbers play a pivotal role in this arrangement. The Aufbau principle, Pauli exclusion principle, and Hund’s rule guide the distribution of electrons among orbitals:
- Aufbau Principle: Electrons fill orbitals starting with the lowest energy levels first.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons with opposite spins.
- Hund’s Rule: Electrons occupy degenerate orbitals singly before pairing up to minimize electron repulsion.
By following these principles, we can determine the electron configuration of an atom, which affects its chemical properties and bonding behavior.
Impact on Chemical Properties
The electron configuration of an atom determines its chemical properties, including reactivity, bond formation, and overall stability. Here are some key ways quantum numbers influence chemical properties:
- Valence Electrons: The outermost electrons, or valence electrons, determine an atom’s chemical reactivity. Elements with similar valence electron configurations exhibit similar chemical behaviors.
- Periodic Trends: Quantum numbers help explain periodic trends such as atomic radius, ionization energy, and electron affinity. For example, elements in the same group have similar valence electron configurations, leading to comparable chemical properties.
- Bond Formation: The arrangement of electrons in orbitals influences how atoms form bonds. For instance, the hybridization of orbitals (sp, sp², sp³) affects the shape and type of chemical bonds formed in molecules.
- Magnetic Properties: The spin quantum number affects the magnetic properties of an atom. Unpaired electrons contribute to paramagnetism, while paired electrons result in diamagnetism.
Examples in the Periodic Table
- Noble Gases: Noble gases, such as helium and neon, have a complete outer electron shell. Their stable electron configuration makes them chemically inert.
- Transition Metals: Transition metals, like iron and copper, have partially filled d orbitals. This leads to variable oxidation states and complex bonding behavior.
- Halogens: Halogens, such as fluorine and chlorine, have seven valence electrons. They are highly reactive and readily form bonds to achieve a stable octet configuration.
Conclusion
Quantum numbers are crucial for understanding the arrangement of electrons in atoms and their impact on chemical properties. They dictate the electron configuration, which influences an element’s reactivity, bonding behavior, and overall chemical behavior. By studying quantum numbers, chemists can predict and explain the properties of elements and their compounds, advancing our knowledge of chemistry and materials science.